Listen to the article
Yes, A lead acid battery has a freezing point. It could become damaged or ruined. But under what circumstances will a flooded lead acid battery freeze (like those in your car or truck, tractor, riding mower, ATV, boat, generator, motorcycle, etc.)?
I’ve included a lead acid battery freeze-temperature (versus state-of-charge) chart below…
Putting it simply, a completely depleted ‘dead’ lead acid battery will freeze at 32°F (0°C). When a lead acid battery is fully discharged, the electrolyte inside is more like water, so it will freeze.”
(Jump down to chart)
What happens when a lead acid battery electrolyte physically freezes? Likely, it will become irreparably ruined to a sufficient extent. The ice that forms will expand and press on the internal cells and plates (and bulge the wall/case of the battery itself).
The case may even crack, resulting in leaks. Given internal physical tolerances, some (or all) of it may ‘short out’ inside. The battery may never hold a proper charge (or any charge) again.
However, a well-charged lead acid battery in good condition will not freeze in practical use. However, the less charged it is, the more susceptible to freeze damage. Even for a fully charged lead acid battery, there’s still a point of freezing. But those temperatures are extremely cold and you likely will not ever experience that cold (keep reading).
The problem arises when your battery is only partially charged or is no longer in good condition. This is when you might run into trouble during the cold winter months.
A little more detail… The exact numbers vary a bit, depending on a few factors. A fully charged (lead acid) battery will freeze. But not until temperatures drop to -94°F (-70 °C)! That’s not going to happen anywhere here on earth, right?!
Can a flooded battery freeze?
A battery can only freeze if it is left in a state of partial or complete discharge.
As the state of charge in a battery decreases, the electrolyte becomes more like water, and the freezing temperature increases.
The freezing temperature of the electrolyte in a fully charged battery is -92º F (-69º C). At a 40% state of charge, electrolytes will freeze if the temperature reaches approximately 16º F (-9º C).
~TrojanBattery Company
Important >> The less charge on the lead acid battery, the more susceptible it is to freezing.
I built a chart that cross-references battery state-of-charge with the approximate temperature at which the battery will freeze. This is for lead acid type batteries, such as car batteries or those typically installed in lawn tractors, ATVs, snowmobiles, and maybe even your camper.
Lead Acid Battery Freeze Chart
Temperature vs State of Charge
How to read the chart:
- If your lead-acid battery is only half charged (50% state of charge), it can freeze solid at −4°F. At that point, permanent internal damage is likely, and replacement is usually the only option.
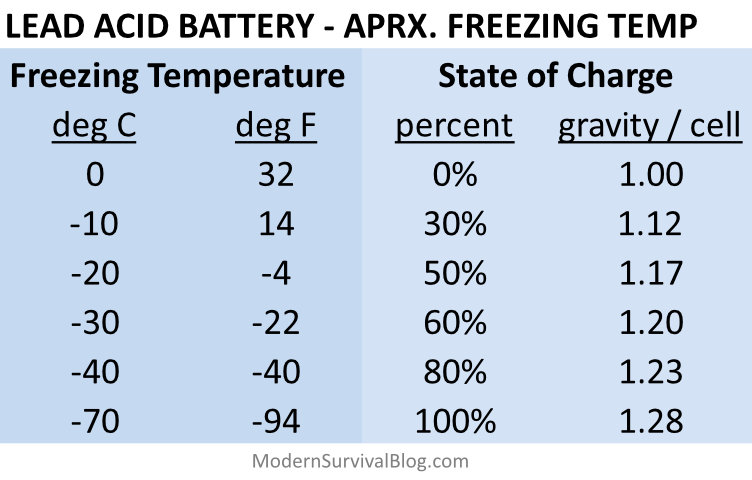
To put it another way, a lead acid battery’s freezing point rises rapidly as charge drops: about −40°F at 80% charge, −22°F at 60% charge, and just 14°F at 30% charge.
Frequently Asked Questions About Battery Freezing
A fully discharged lead acid battery can freeze at 32°F (0°C). At this point, the electrolyte is mostly water, which explains why freezing occurs so easily. A fully charged battery will not freeze until temperatures approach −90°F, which is extremely unlikely in real-world conditions.
No, a healthy fully charged lead acid battery will not freeze under normal winter conditions. Freezing typically occurs when the battery is partially discharged or has internal degradation.
In most cases, no. When electrolyte freezes, it expands and can permanently damage plates and internal separators, often ruining the battery.
Keep the battery fully charged, eliminate parasitic loads, and use a smart trickle charger during long cold-weather storage.
Because freezing risk rises sharply as state of charge drops, the simplest and most reliable winter protection is maintaining a full charge with a temperature-compensated smart trickle charger. For this reason, I personally use and recommend the Battery Tender Plus and Battery Tender Junior.


As an Amazon Associate I earn from qualifying purchases.
(no extra cost) Thank you
Battery Tender Plus – 1.25 Amp
Battery Tender Junior – 750 mA


The following article provides a better understanding of the battery’s state of charge (approx.. corresponding open-circuit voltage). There are caveats to these measurements, which are clearly explained in the article:
[ Read: Battery State-Of-Charge Chart ]
I often see temperatures below zero during the coldest winter months. The coldest I’ve experienced here was -32°F. During some periods of the winter months, I may experience morning temperatures around 20 below zero, so I need to be attentive.
I lost two 12-volt batteries several years ago in my fifth-wheel camper. Although they had been charged, I forgot to physically disconnect them for the winter. As it turned out, a small parasitic drain constantly drew down the batteries (propane gas detectors and a few other things). The batteries eventually went dead, and then they froze. It was an expensive mistake that I will never make again!
What about the 12-volt batteries on your tractor, lawn mower, ATV, etc., during winter?
Some people remove them and store them inside where it’s warm during the winter. This is a good idea—better safe than sorry, right?
However, you can leave a lead acid battery installed during the winter. But only if the battery is in good condition, there is no parasitic load slowly draining the battery, and it is fully charged. I keep trickle chargers on mine, just in case.
Read the full article here


22 Comments
I’m concerned about the environmental impact of lead acid batteries freezing and potentially leaking, are there any eco-friendly alternatives available?
The freezing temperature of the electrolyte in a fully charged battery is -92º F, which is extremely cold, but what about the freezing temperature of a partially charged battery, how does that affect its performance?
I’m curious about the physical tolerances of the internal cells and plates in a lead acid battery, how do they handle the expansion of ice when the electrolyte freezes?
From what I understand, the ice expansion can cause the case to crack, resulting in leaks and potentially shorting out the battery.
I’ve experienced issues with my generator’s battery freezing during the winter, and I think it’s because I didn’t keep it charged, according to the article, a partially charged battery is more prone to freezing.
I was surprised to learn that a fully charged lead acid battery will not freeze in practical use, but a partially charged one is more susceptible to freeze damage, especially at temperatures around -4°F for a 50% state of charge.
I’m skeptical about the claim that a fully charged lead acid battery will not freeze in practical use, what about extreme cold climates, won’t the battery still be at risk of freezing?
The article mentions that even in extreme cold climates, a fully charged battery is unlikely to freeze, but it’s still important to take precautions to prevent damage.
The article mentions that a little more detail is needed to understand the exact numbers, but it’s clear that the state of charge has a significant impact on the freezing point of a lead acid battery.
The fact that a fully charged lead acid battery won’t freeze until temperatures drop to -94°F is astonishing, I live in a cold climate and I’ve never experienced temperatures that low.
I’ve been using lead acid batteries for my snowmobile, and I’ve noticed that they can be prone to freezing, especially if they’re not properly maintained, thanks for the info on how to prevent freezing.
The article mentions that the Trojan Battery Company states that the less charge on the lead acid battery, the more susceptible it is to freezing, which makes sense given the water-like consistency of the electrolyte.
The chart provided is really helpful in understanding how the state of charge affects the freezing point of a lead acid battery, I noticed that at a 40% state of charge, the electrolytes will freeze at approximately 16º F.
I’ve learned a lot from this article about lead acid batteries and freezing temperatures, thanks for the info, I’ll make sure to keep my batteries charged and maintained during the winter.
I’ve had issues with my car battery freezing in the past, and I had no idea that the state of charge played such a significant role, according to the article, a fully discharged battery will freeze at 32°F, which makes sense given the water-like consistency of the electrolyte.
Yeah, it’s really important to keep your battery charged, especially during the winter months, to prevent damage from freezing.
I’ve been using lead acid batteries for my lawn tractor and ATV, and I had no idea that they could freeze if left in a state of partial or complete discharge, thanks for the info.
The chart is really helpful in understanding how to read the state of charge with the approximate temperature at which the battery will freeze, I’ll make sure to keep an eye on my battery’s state of charge during the winter.
The article provides a lot of useful information about lead acid batteries and freezing temperatures, but I’m still unsure about how to prevent my battery from freezing, are there any tips or recommendations?
I’m curious about the long-term effects of a lead acid battery freezing, will it always result in permanent damage, or can it be repaired?
From what I understand, if the battery freezes, it’s likely to be irreparably ruined, and replacement is usually the only option.
The fact that a lead acid battery’s freezing point rises rapidly as charge drops is really important to understand, especially for those living in cold climates.